- CDSCO MD-42 Registration Certificate is the mandatory license for anyone selling, stocking, or distributing medical devices in India under the Medical Device Rules, 2017.
- It replaces the need for a generic drug license for device-only sellers and requires appointing competent technical staff and maintaining proper storage conditions.
- The license is valid in perpetuity, provided you pay a retention fee every 5 years, ensuring long-term business continuity.
Introduction

Imagine landing a lucrative contract to supply MRI machines or even simple glucometers to a top hospital chain, only to have your shipment stalled or your invoice rejected because you lack a single piece of paper. This is the reality for medical device traders in India today.
Since the enforcement of the Medical Device Rules (MDR), 2017, the days of operating in a regulatory grey area are over. If you are in the business of selling medical devices, having a CDSCO MD-42 Registration Certificate is no longer optional—it is your license to exist. This blog cuts through the legal jargon to explain exactly how to secure your MD 42 license, ensuring your business remains compliant, credible, and profitable.
What is a CDSCO MD-42 Registration Certificate?
The CDSCO MD-42 Registration Certificate is the official license granted by the State Licensing Authority (SLA) that authorizes an entity to sell, stock, exhibit, or offer for sale or distribute medical devices in India. Before 2017, medical devices were often lumped together with pharmaceutical drugs. The MDR, 2017 created a specific identity for them, recognizing that a stent needs different regulation than a paracetamol tablet.
Difference Between Form MD-41 (Application) and MD-42 (Grant)
It is crucial to distinguish between the two forms to avoid confusion during your application:
- Form MD-41: This is the application form you fill out and submit to the licensing authority.
- Form MD-42: This is the actual license (certificate) you receive after approval.
Think of MD-41 as your exam paper and MD-42 as your degree.
Scope of MD-42: Sale, Stock, and Distribution of Medical Devices
This license covers the entire downstream supply chain. Whether you are storing cardiac stents in a warehouse or selling digital thermometers at a retail counter, if the product is a "Notified Medical Device," this license applies. It ensures that the product quality is maintained from the factory gate to the patient's bedside.
A CDSCO MD-42 license is a regulatory approval required for the sale and distribution of medical devices in India. It is granted in Form MD-42 after submitting an application in Form MD-41, ensuring compliance with storage and technical staffing standards under MDR, 2017.
Who Needs a CDSCO MD-42 License?
Any business entity positioned in the supply chain between the manufacturer and the end-user requires this license. Specifically, the CDSCO MD-42 License is mandatory for distributors, wholesalers, stockists, and retailers who store, exhibit, or sell notified medical devices—ranging from simple consumables like IV sets to complex implants.
Medical Device Distributors and Wholesalers
If your core business is B2B supply—buying from manufacturers and selling to hospitals or other retailers—you are the primary candidate for this license.
Retailers and Pharmacies Selling Medical Devices
While traditional pharmacies holding Form 20/21 Drug Licenses are technically covered, dedicated medical device outlets (e.g., shops selling only orthopaedic supports or hearing aids) must obtain the MD 42 Registration online.
Importers Stocking Devices for Sale in India
Importers already need an MD-14 Import License to clear customs. However, if that importer also acts as the distributor storing goods in a domestic warehouse for further sale, they need the CDSCO Wholesale medical device license (MD-42) for that specific premise.
Is CDSCO MD-42 License mandatory?
yes. If you are engaged in the business of selling, stocking, exhibiting, or distributing any product officially classified as a "Medical Device" under Indian law, holding a CDSCO MD-42 License is not a choice—it is a legal compulsion.
Legal Implications of Selling Devices Without MD-42
Yes, it is mandatory. Operating without it is a violation of the Drugs and Cosmetics Act, 1940.
- Seizure of Stock: Drug Inspectors can seize your entire inventory.
- Prosecution: You risk facing legal prosecution, fines, and immediate closure of business operations.
- Supply Chain Blacklist: Compliant hospitals and manufacturers will refuse to do business with unlicensed vendors to protect their own liability.
List of Exempted Devices (If any)
Currently, almost all medical devices notified by the government fall under this purview. While some very low-risk items might have relaxed norms, it is dangerous to assume exemption. Always verify the status of your specific device with a consultant.
Who can apply for MD-42 License?
The application for a CDSCO MD-42 Registration Certificate is open to any legal business entity in India that intends to sell medical devices. However, the "applicant" (the business) must prove it has the technical capability to handle these products safely. The Central Drugs Standard Control Organization does not issue this license to just anyone with a warehouse; you need qualified supervision.
Registered Pharmacists vs. Competent Persons
You don't necessarily need a pharmacist if you are only selling medical devices. You can appoint a "Competent Person."
- Registered Pharmacist: Automatically eligible.
- Competent Person: A graduate in Science/Pharmacy with 1 year of relevant experience.
Proprietorships, Partnerships, and Private Limited Companies
Any legal entity can apply. The key is that the "applicant" (the firm) must own or rent the premises where the license is sought.
What are the Eligibility Criteria for MD-42 License?
To get your Medical Devices Registration for sales, you must prove you are capable of handling these sensitive products.
Qualification Requirements for Competent Staff
The person in charge of sales must be:
- A Registered Pharmacist, OR
- A Graduate in a recognized University (Science/Pharmacy/Medicine) with one year of experience in dealing with medical devices.
Minimum Experience Required for Medical Device Handling
If you are hiring a science graduate, that "one year of experience" must be documented. A simple experience letter from a previous employer (who holds a valid license) usually suffices.
What is the Requirement for MD-42 License?
The Drug Inspector (DI) will physically verify these during the inspection.
Carpet Area and Premises Layout Standards
While the law doesn't specify a rigid square footage like it does for drugs (e.g., 10 sq meters), the premises must be "adequate" to store the volume of stock without overcrowding. It should be a permanent structure (brick and mortar), not a temporary shed.
Storage Facilities: Racks, Pallets, and Hygiene
- Pallets: Goods must not be kept directly on the floor.
- Hygiene: The area must be dust-free, pest-controlled, and clean.
- Separation: Distinct areas for "Saleable Stock" and "Expired/Damaged Stock" are mandatory.
Cold Chain Maintenance for Temperature-Sensitive Devices
If you sell IVD reagents or certain implants, you must have a refrigerator with a temperature log (data logger) to prove you maintain the 2°C–8°C range.
Key Requirements for Grant of Registration Certificate (MD-42)
Getting your CDSCO MD-42 Registration Certificate is not just about submitting PDFs; it is about proving to the Licensing Authority that you run a safe, professional operation. The government treats medical devices as life-saving tools, and they expect your facility to reflect that seriousness. The Drug Inspector (DI) will verify these three critical operational standards before—and after—granting your license.
Technical Staff Availability during Working Hours
The Competent Person named in your application must be physically present during business hours. If the inspector visits and the staff is missing, your application will be rejected.
Record Keeping and Traceability of Devices
You must maintain records that allow for "Traceability." If a manufacturer recalls a batch of pacemakers, you must be able to tell exactly which hospital or patient received them.
Separation of Expired or Damaged Goods
A dedicated "Quarantine Area" for expired goods is a red-flag check for inspectors. If they find expired goods mixed with fresh stock, it is an instant violation.
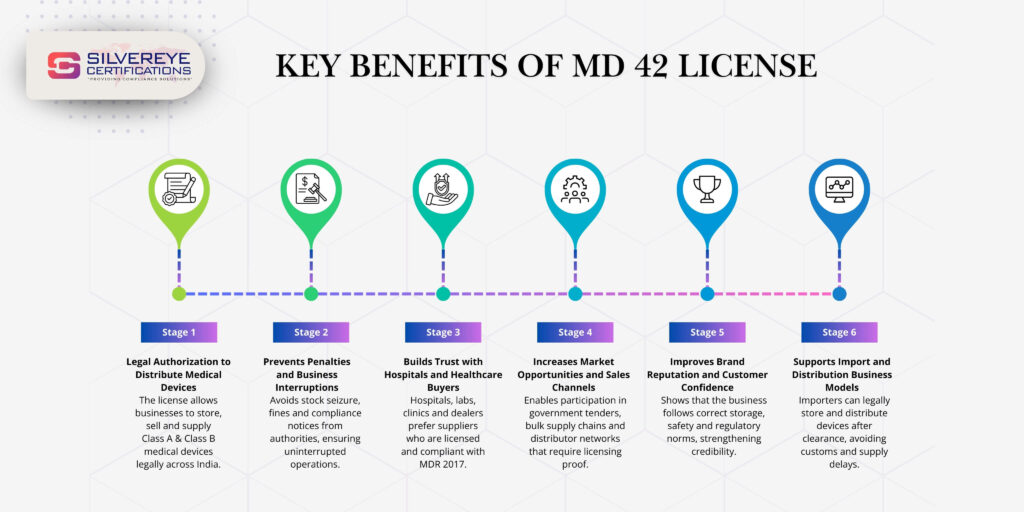
Benefits of MD-42 Registration Certificate
Many business owners view regulatory compliance as a cost, but in the current Indian healthcare market, your CDSCO MD-42 Registration Certificate is actually a high-value asset. It separates the serious, long-term players from the unorganized "box movers." Beyond just keeping the law away, here is how this license actively helps you grow your business.
Legal Protection and Business Credibility
Holding an MD 42 license protects you from regulatory harassment. It signals to the market that you are a legitimate player, not a fly-by-night operator.
Access to Government Tenders and Hospital Supply
You cannot bid for government tenders (GeM portal) or supply to major corporate hospitals (Apollo, Max, Fortis) without this license. It is a standard "Technical Qualification" criterion.
Building Trust with International Manufacturers
Global brands like Medtronic, Siemens, or Roche will only appoint distributors who are fully compliant. Your Medical Devices Certification is your ticket to these premium partnerships.
Which Documents are required for an MD-42 Registration Certificate?
Prepare these before you even open the online portal.
KYC and Constitution Documents of the Firm
- PAN Card & GST Certificate.
- Certificate of Incorporation / Partnership Deed / MOA & AOA.
Proof of Ownership or Rent Agreement for Premises
- Valid Rent Agreement (Commercial usage).
- Electricity Bill of the premises.
- NOC from the owner.
Educational and Experience Certificates of Technical Staff
- Degree Certificate.
- Registration Certificate (for Pharmacists).
- Experience Letter (for Competent Persons).
- Appointment Letter and Acceptance Letter.
Site Layout Plan and Blueprint
A clear layout showing the entrance, storage racks, cold storage area, and quarantine area.

What is the process of the MD-42 Registration Certificate?
While the government has digitized the application, the CDSCO MD-42 Registration Certificate process is a hybrid of online filing and offline verification. It is not as simple as "upload and print." The State Licensing Authority (SLA) scrutinizes every detail to ensure your facility is genuine. Here is the step-by-step roadmap we follow for our clients:
Step 1: Document Compilation and Verification
Gather all documents. Ensure the name on the rent agreement matches the GST and the application exactly.
Step 2: Filing Application via Online Portal (Sugam/NSWS)
MD-42 is issued by the State Licensing Authority (SLA). While some states use NSWS/SUGAM, most have their own portals (e.g., XLN for Gujarat/North India).
Step 3: Inspection by Drug Inspector (DI)
Once the application is reviewed, a Drug Inspector will visit your premises. They will check the layout, cleanliness, and interview your technical staff.
Step 4: Query Resolution and Grant of License
If the DI raises queries (e.g., "Add more racks"), you must comply. Once satisfied, the Licensing Authority issues the CDSCO MD-42 Registration Certificate.

CDSCO MD-42 License Cost and Timeline
Here is the detailed cost and timeline table based on the official Medical Device Rules, 2017.
The following fee structure is prescribed under the Second Schedule of the Medical Device Rules, 2017. Please note that these are the statutory government fees paid via the online portal (SUGAM/NSWS). Professional consultancy fees for documentation and audit preparation are separate.
| Component | Form Number | Govt. Fee (INR) | Official Timeline* | Validity |
| Application for Grant | Form MD-41 | ₹3,000 | 10 Days (After satisfying all conditions) | Perpetual (Lifetime) |
| Retention Fee | N/A | ₹3,000 | Due every 5 years | Keeps license active |
| Duplicate Copy | N/A | ₹500 | 7-10 Days | N/A |
While the official rule states the license shall be granted within 10 days after the State Licensing Authority is satisfied with your inspection report, the practical end-to-end timeline (including document review, query resolution, and Drug Inspector's visit) is typically 30 to 45 days. Source: CDSCO Medical Device Rules, 2017 - Second Schedule
CDSCO MD-42 License Validity and Renewal
Understanding the 5-Year Validity / Perpetual Retention Rule
The MD 42 license is valid in perpetuity (lifetime). It does not expire. However, you must pay a retention fee every 5 years to keep it active.
Process for Paying Retention Fees
You must pay the ₹3,000 retention fee via the online portal before the completion of the 5-year block. Failure to pay leads to automatic suspension of the license.
Common Reasons for Rejection in MD-42 License
Inadequate Storage or Carpet Area
Applying for a wholesale license in a small, cramped 100 sq. ft. room often leads to rejection.
Mismatch in Technical Staff Documents
If your competent person’s experience letter looks fake or doesn't match their degree timeline, the application will be flagged.
Failure to Produce Records During Inspection
If the inspector asks for your purchase records or temperature logs and you cannot produce them, it shows a lack of "Good Distribution Practices."
Why Choose Silvereye Certifications as Your CDSCO Consultant for MD-42 Registration?
Navigating the CDSCO MD-42 Registration Certificate process can be tedious. One small error can delay your business by months.
Our Expertise in Medical Device Rules 2017
We specialize in medical device regulations. We stay updated on every notification and amendment so you don't have to.
End-to-End Documentation and Inspection Support
We don't just file forms. We conduct pre-audit checks of your facility to ensure you pass the Drug Inspector’s visit with flying colors.
100% Client Satisfaction and Success Rate
We have helped hundreds of distributors and retailers secure their MD 42 license seamlessly.
Conclusion
The medical device market in India is booming, but so is the regulatory oversight. Obtaining your CDSCO MD-42 Registration Certificate is the first step toward building a sustainable, compliant business. Don't view it as a hurdle; view it as a competitive advantage that separates you from unorganized players.
If you are ready to secure your MD 42 Registration online without the headache of rejections and delays, our team is here to help.
Contact Silvereye Certifications Today for a Free Consultation
Frequently Asked Questions
Is a Registered Pharmacist mandatory for an MD-42 License?
No. While a Pharmacist is automatically eligible, you can also appoint a "Competent Person." This person must be a Graduate in Science or Pharmacy with at least one year of documented experience in dealing with medical devices.
What is the difference between Form 20B/21B and Form MD-42?
Form 20B/21B is the old wholesale drug license for pharmaceuticals. Form MD-42 is the specific registration certificate required under the Medical Device Rules, 2017 for selling medical devices. If you sell devices, you specifically need MD-42.
Is the MD-42 License valid for a lifetime?
Yes, the license is valid in perpetuity. However, you must pay a License Retention Fee of ₹3,000 every 5 years to keep it active. If you miss this payment, the license is suspended.
Can I operate my medical device business from a residential address?
Generally, no. The Drug Inspector requires a commercial or mixed-land use premise. You must have a separate entrance and adequate storage space (racks/pallets) that is not part of a living area.
How long does it take to get the MD-42 Registration Certificate?
The official timeline is short (10 days after inspection), but practically, the entire process—including document review, portal submission, and the Drug Inspector's visit—takes about 30 to 45 days.
Do importers with an MD-14 license also need MD-42?
Yes. Form MD-14 allows you to enter the goods into India (Customs clearance). To stock and sell those goods from your Indian warehouse to distributors or hospitals, you need a valid CDSCO MD-42 Registration Certificate for that warehouse.
Can I sell medical devices online (Amazon/Flipkart) with this license?
Yes. E-commerce platforms now mandatorily require you to upload your MD-42 Registration Certificate before they list medical products like glucometers, BP monitors, or nebulizers.
What is the government fee for MD-42 Registration?
The statutory government fee is ₹3,000, payable via the online portal (SUGAM or state-specific system). This does not include professional consultancy fees.
What happens if I get caught selling devices without a license?
It is a violation of the Drugs and Cosmetics Act. The State Drug Controller can seize your stock, impose heavy fines, and launch criminal prosecution for selling unauthorized medical goods.
Do I need to maintain cold chain storage?
Only if you sell products specified as temperature-sensitive by the manufacturer (e.g., IVD reagents, certain glues/sealants). In such cases, a refrigerator with a temperature log is mandatory for the MD-42 license approval.















